Rotasiil Clinical Trial Results

Results from a Phase 3 efficacy study in India of the Serum Institute of India Pvt. Ltd.’s rotavirus vaccine BRV-PV (known as ROTASIIL®) were published in the journal Vaccine. The study showed the vaccine to be safe, well tolerated, and to provide significant efficacy against severe rotavirus gastroenteritis. Read the full press release.
Related fact sheets below offer further technical detail and context in English and Hindi. Media statements from international experts (in English) celebrate the study results and welcome the potential addition of ROTASIIL to the global rotavirus vaccine portfolio.
VACCINE PUBLICATION (English)
PRESS RELEASE ON STUDY RESULTS
English (PDF 219 KB)Hindi (PDF 153 KB)
FACT SHEETS
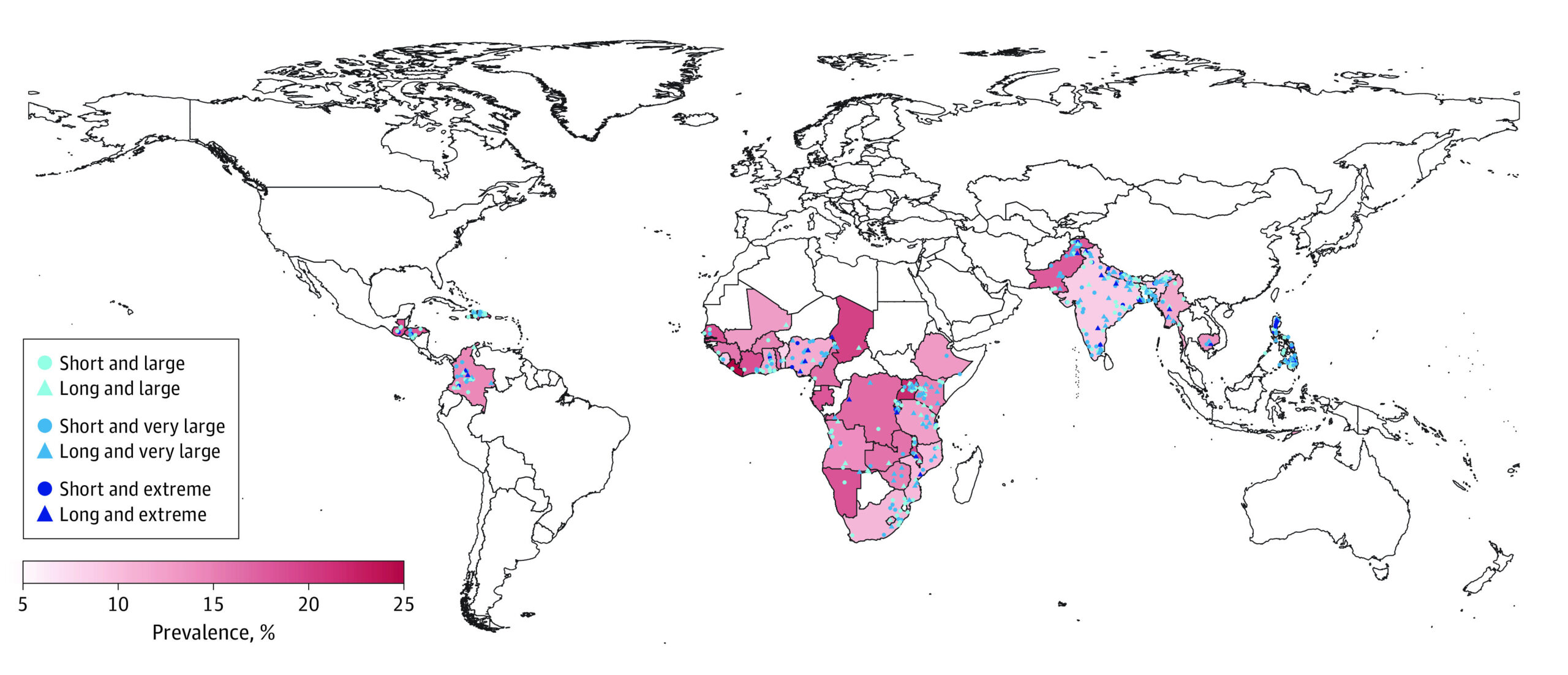
“Rotavirus disease burden in India”
In 2013, an estimated 47,100 rotavirus deaths occurred in India, 22 percent of all rotavirus deaths that occurred globally.
English (PDF 130 KB)Hindi (PDF 172 KB)
“The need for rotavirus vaccines in India: Understanding efficacy and impact”
Rotavirus is particularly threatening in India. The prevention of the disease through vaccines is an essential element to save lives from this lethal form of diarrheal disease.
ADDITIONAL MEDIA STATEMENTS
To view the full list, scroll through the statements below or click here (PDF 83 KB).
“It is important to have a new approach to look at rotavirus vaccine efficacy. Rotavirus vaccination in developing countries has always struggled with interpretation of efficacy through use of markers of seroconversion or reduction of death in the community. Both are erroneous because of babies being breastfed as well as high infant mortality under five years of age in certain geographic regions of India. Better indicators have been surrogate indicators, which reflect fewer admissions of children to the hospital related to rotavirus as well as a reduced stay in the hospital due to the same. These markers have worked well in Mexico and El Salvador.”
Prof. N.K. Ganguly, Former Director General, Indian Council of Medical Research; Visiting Professor of Eminence, Policy Center for Biomedical Research, Translational Health Science & Technology Institute, Faridabad, India
“India now has another rotavirus vaccine that is manufactured indigenously. This is a win for the children of the country as it will support the Government of India’s efforts to roll out the rotavirus vaccine across the country through the Universal Immunization Programme.”
Dr. M.K. Bhan, Former Secretary, Department of Biotechnology, Government of lndia
“I am delighted that India has another rotavirus vaccine. Scientific studies have shown that rotavirus vaccines are making a major public health impact. Swift and significant declines in hospitalizations and deaths due to rotavirus and all-cause diarrhea have been observed in many of the countries that have introduced rotavirus vaccines into their national immunization programs.”
Dr. Gagandeep Kang, Executive Director of Translational Health Science & Technology Institute, Faridabad, India, and member of the ROTA Council
“Rotavirus is the most common cause of diarrhea and one of the leading cause of under-five child mortality. Rotavirus accounts for approximately 40 percent of all diarrhea cases requiring treatment. Rotavirus disease cannot be treated with antibiotics or other drugs. Rehydration therapy is an important part of treating dehydration due to diarrheal diseases, including rotavirus. Parents often bring children with severe diarrhea to the hospital at the last minute. Since diarrhea in infants is associated with teething, families do not often take the situation seriously. With the rotavirus vaccines now available in the Universal Immunization Programme, the high disease burden will certainly see a decline. Also immunized children are likely not to need hospitalization.”
Dr. Naveen Thacker, President of Asia Pacific Pediatric Association (APPA) and Strategic Advisor on Immunization for International Pediatric Association (IPA)
“In 2013, an estimated 47,100 rotavirus deaths occurred in India, which accounted for 22 percent of all rotavirus deaths that occurred globally. A new indigenous vaccine is a welcome step for children in India, and it will help save children from death and disability. The efficacy of the vaccine compares well with the efficacy of the already licensed rotavirus vaccines in clinical studies conducted in low resource settings, particularly for preventing the most severe cases of rotavirus diarrhea. Another advantage of this vaccine, in the future, is that it will be heat stable—therefore it can be used outside the cold chain.”
Dr. Pramod Jog, President, Indian Academy of Pediatrics, 2016 and Standing Committee Member, International Pediatric Association (IPA), 2016-19
“Rotavirus is found in all countries including India. Regardless of hygiene practices or access to clean water, nearly every child in the world is at risk of infection with rotavirus before the age of five years. Rotavirus vaccines are an important part of an integrated prevention and treatment strategy to control diarrheal disease. Other elements of this strategy include oral rehydration therapy; zinc treatment; exclusive breastfeeding; proper nutrition; safe water; and improved sanitation and hygiene practices. India needs indigenous rotavirus vaccines to ensure that children are saved and have a shot at a full and healthy life.”
Dr. Dipika Sur, Principal Investigator for the Global Enteric Multicenter Study (GEMS) in India and Former Senior Scientist, Indian Council for Medical Research