
What would success look like for a Shigella vaccine?
Researchers are working to advance a vaccine against Shigella. Here, one of the candidates is being prepped for use in a clinical study. Photo: PATH.
This post originally appeared on the PATH website.
Shigella remains an underappreciated child health challenge in communities that lack access to safe drinking water and sanitation. As one of the leading bacterial causes of childhood diarrhea, especially dysentery, it contributes to stunting, growing antimicrobial resistance, and long-term economic consequences.
PATH’s approach to vaccine development begins with an informed conception of the ideal product. Since there are no vaccines against Shigella yet available, now is an opportune time to ask some crucial questions. What could be the potential impact of Shigella vaccines? Will there be demand for these vaccines among national stakeholders? What attributes are most salient?
Like the injectable next-generation rotavirus vaccine value proposition before it, PATH’s Shigella vaccine public health value proposition comprehensively considered these questions and others using multiple tools, such as economic analyses, data synthesis, expert convenings, and stakeholder interviews. Our findings hold broad implications for immunization funders and developers of Shigella, and, in some cases, other enteric vaccines.
The impact of a vaccine that could prevent stunting
Though the link between severe Shigella illness and stunting is well established, PATH synthesized new evidence suggesting that both moderate and less-severe diarrhea caused by Shigella contribute to long-term growth shortfalls. We then used the expanded burden figures as inputs for our vaccine impact models, which greatly increased the estimated cost-effectiveness of a Shigella vaccine that could prevent stunting as an outcome.
The second model extends the cost-effectiveness findings by estimating a potential Shigella vaccine’s impact on adult wage earning. It suggests the potential benefit of a Shigella vaccine that reduces stunting is eight times the estimated costs in 102 countries. We believe this new and unique framework for measuring vaccine impact on economic productivity via reduced stunting could hold relevance for estimating impact of other vaccines.
Measuring vaccine protection against long-term health impacts
The models rely on the assumption that a Shigella vaccine will prevent stunting by preventing symptomatic diarrhea—but this remains an important, unanswered question.
As researchers advance Shigella vaccine candidates, measuring their long-term impact on stunting and antimicrobial resistance (AMR) should be among the clinical end points. This evidence is necessary to refine model results, understand the full value of vaccines that reduce stunting, and generate greater demand, since national stakeholders interviewed in our feasibility and acceptability study in five countries expressed strong preference for a vaccine that could reduce stunting (and AMR).
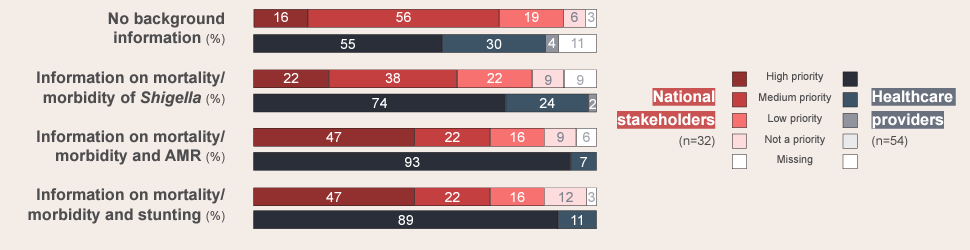 The overall priority of a Shigella vaccine rose among participants as they were provided with progressively more country-specific information about the possibility of preventing longer-term issues related to Shigella, notably reducing AMR and stunting.A streamlined approach to combination vaccine development
The overall priority of a Shigella vaccine rose among participants as they were provided with progressively more country-specific information about the possibility of preventing longer-term issues related to Shigella, notably reducing AMR and stunting.A streamlined approach to combination vaccine development
In addition to a vaccine that can reduce stunting, national stakeholders also voiced a strong preference for Shigella-containing combination vaccines. Likewise, in commercial markets such as those for military personnel and in travel medicine clinics, a combination approach that also offers protection against other priority pathogens may have greater uptake.
This represents a growing trend as many new vaccines against multiple pathogens are in development. In June 2022, PATH convened an independent panel of 34 academic, industry, philanthropic, and global health experts to discuss the potential barriers to the development, licensure, policy recommendations, and introduction of a Shigella-containing combination vaccine.
The experts concluded that current policy recommendations and financial incentives are not attuned to the special risks associated with combination vaccine development. In addition, the clinical development and regulatory barriers to combination vaccine licensure are formidable.
The availability of new innovative technologies that can accommodate multiple vaccine pathogens, such as the mRNA platform, could help catalyze the needed pathways to efficiently develop combination vaccines.
Evidence dissemination and improved local data
Stunting and AMR are high-priority issues for national stakeholders. Yet while their background awareness of Shigella diarrhea is high, it takes lower priority, indicating the need for stronger awareness of Shigella’s connection to these long-term consequences. Improved Shigella surveillance to fill gaps in local-burden data is also important for decision-making at the national and subnational levels.
The takeaway is the need to cascade the compelling evidence already available more strongly from the global to the national level. PATH’s Defeat Diarrheal Disease Initiative, which began its work when rotavirus vaccines were just becoming available, can serve as a model for engaging global and national advocates with evidence-based advocacy tools to generate awareness and investment in diarrhea prevention and control in settings where the solutions remain out of reach for too many children.
Some of the participants in the feasibility and acceptability study rightly noted that Shigella can be prevented with clean water, hygiene, and sanitation; the danger is in thinking that the problem is solved. It’s incumbent upon researchers and advocates to communicate about the ways in which additional new vaccines could add value.
Vaccines are already among the world’s most cost-effective interventions. We hope researchers and funders use these findings to help make Shigella vaccines a reality.


