
A LAMP to light the way: A rapid diagnostic test for ETEC and Shigella diarrhea
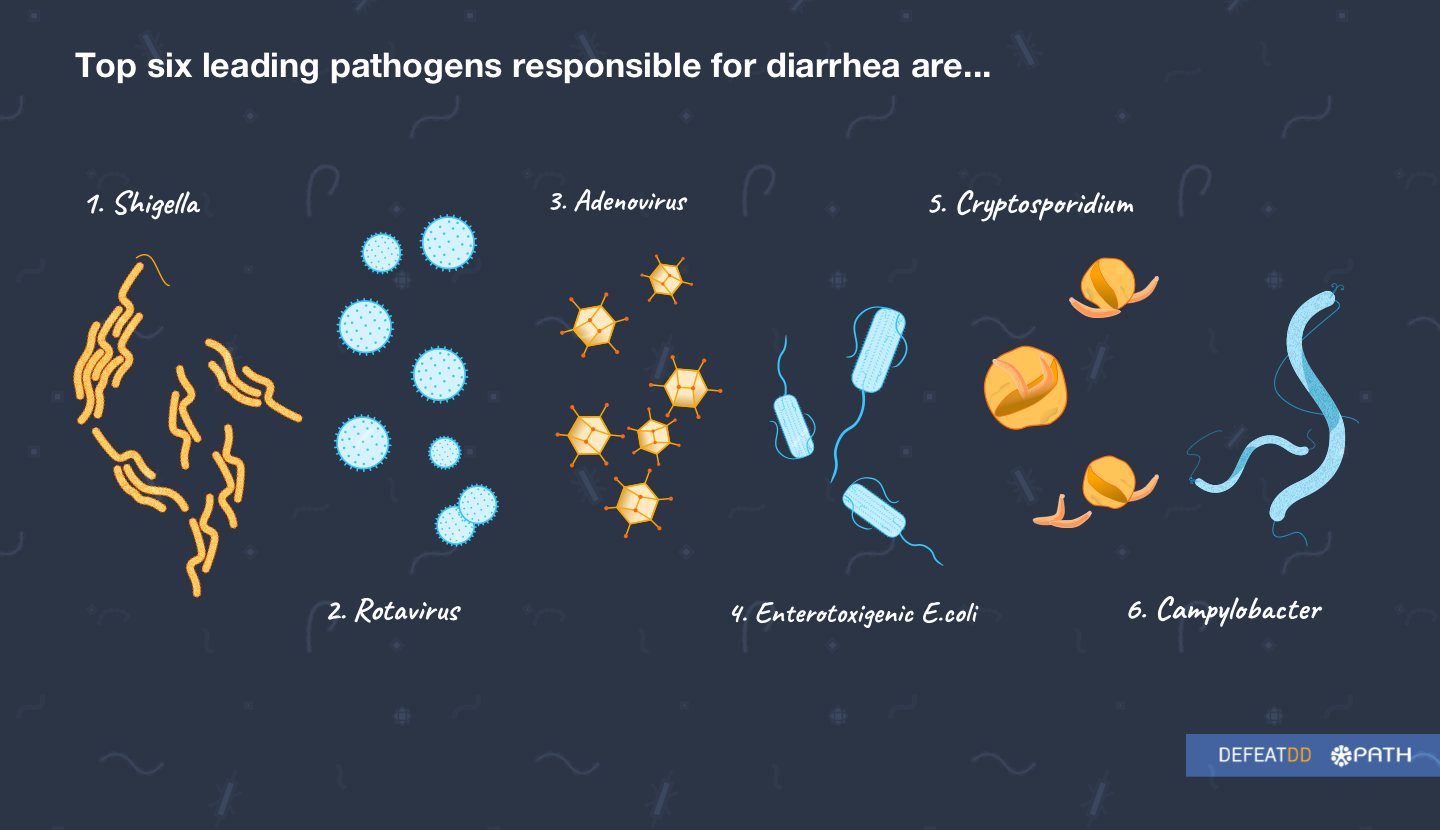
Enterotoxigenic E. coli (ETEC) and Shigella are leading bacterial causes of diarrhea. Experts have long suspected that they are responsible for a large proportion of the global diarrheal disease burden. However, morbidity and mortality estimates of ETEC and Shigella have fluctuated over recent years, which has led to uncertainty in their relative public health importance. One of the main reasons for this fluctuation is a lack of a simple diagnostic test that can be applied in low- and middle-income country settings to get accurate disease burden data.
A simple new rapid diagnostic test for ETEC and Shigella diarrhea may prove to be a game changer. DefeatDD’s Laura Kallen spoke with microbiologist and molecular epidemiologist Dr. Subhra Chakraborty, who led the work on developing this new test, to learn more.
Laura: Could you tell me about your background and how you became interested in ETEC and Shigella diagnostics?
Subhra: I’m a microbiologist and molecular epidemiologist, and I’ve been working in diarrheal diseases for around 20 years. While working in the field in diarrhea-endemic countries, I realized how difficult—and in some cases impossible—it is to apply the currently available diagnostic methods to detect enteric pathogens. ETEC and Shigella are significant, WHO-priority pathogens for vaccine development. With my background, I felt like I was in a unique position to understand what is needed as a public health priority and what is feasible to develop in the lab.
Laura: What are the problems with the current diagnostics?
Subhra: The current diagnostic tests for ETEC, Shigella, and other enteric pathogens are either culture-based or use sophisticated molecular diagnostics, which are time-consuming and not feasible outside a laboratory or without trained technicians. The culture-based tests are not sensitive enough to reflect the true burden of the disease, and they take three to four days to get the results. Molecular tests like PCR are sensitive, but complicated. You need commercial DNA extraction kits and a trained technician, which is often not feasible in the settings with the greatest burden of diarrhea.
Laura: So, how does the new rapid diagnostic work?
Subhra: I had this idea of developing a simple test, but the main challenge was to simplify the stool preparation process. So that’s what we did.
We made the sample preparation very simple—it can be done within 6 minutes. Everything you need is in the kit. It uses a DNA amplification method called LAMP, which stands for LOOP-mediated isothermal amplification. After 40 minutes the reader gives you the results as positive or negative.
We call the test Rapid LAMP-based Diagnostic Test, or RLDT. It took a long time—since 2014—to come to this point where RLDT works really well.
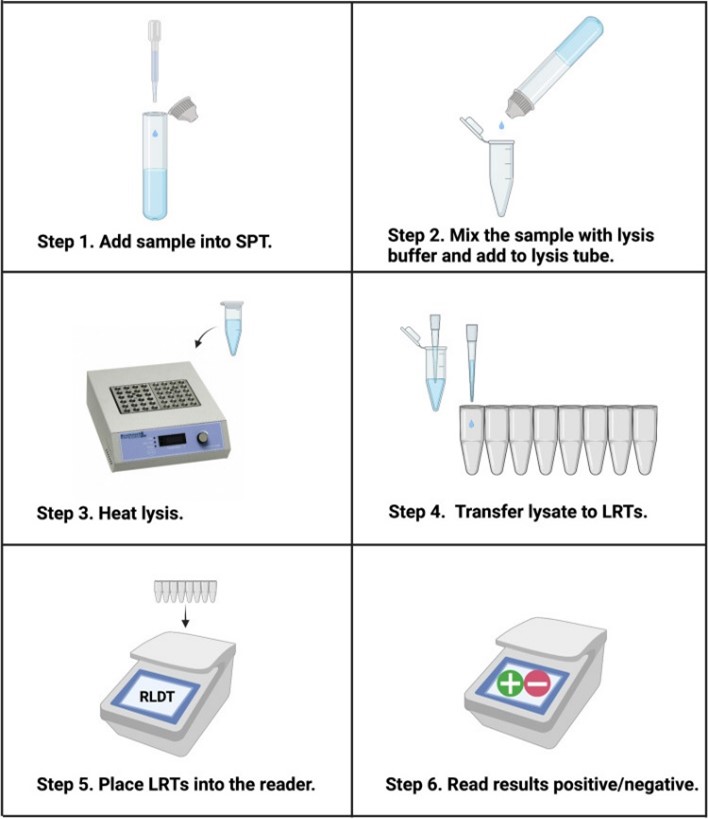 Image: Fig. 1 from https://pubmed.ncbi.nlm.nih.gov/35089927/
Image: Fig. 1 from https://pubmed.ncbi.nlm.nih.gov/35089927/
Laura: Is this new test as accurate as the existing diagnostics?
Subhra: The two things we test for are sensitivity and specificity. For sensitivity, the lowest detection limit tells us the lowest amount of pathogen where it would always be positive. We found this test’s sensitivity is comparable to the test used in the GEMS and MAL-ED studies (TaqMan qPCR array). And for specificity, culture is the gold standard, and RLDT results matched very well with culture results.
Laura: What are the benefits of this new rapid test over the existing diagnostics of either culture or PCR?
Subhra: Mainly, the RLDT is simple and rapid. It works without cold chain, trained personnel, and almost no electricity. Everything is provided in the kit. Additionally, at around $3 per target, it’s inexpensive.
We also see RLDT as having great potential for use at the point of care. In the era of increasing antibiotic resistance, informed decision-making on the use of antibiotics is crucial. With a simple point-of-care test, these pathogens could be detected and treated as required.
Laura: So, what’s next? What are the next steps for this test?
Subhra: We’re really excited about this assay. We’ve done field evaluations with RLDT in India and Zambia and found encouraging results, which we expect to be published soon. Based on those results, we’re currently implementing the assay for surveillance studies in Zambia. We are also looking at the possibility of applying it in rural hospitals in Bangladesh as a point-of-care diagnostic.
Additionally, I see this test ultimately as not just an ETEC/Shigella diagnostic, but as a platform for other enteric pathogens. I’m currently working on an enteric pathogen panel with all the main enteric pathogen targets for diarrhea. Stay tuned!


