
Estimating impact of a novel rotavirus vaccine delivered at birth

More than 300 rotavirus vaccine superstars gathered at the International Rotavirus Symposium in Bali, Indonesia, earlier this year. Photo: PATH/Hope Randall.
Rotavirus remains a common cause of severe diarrhea in young children in Indonesia. Last week, in a strong step forward for child health, the country introduced rotavirus vaccines nationwide, which will greatly reduce disease burden.
While the country celebrates this milestone, work continues behind the scenes to find ways to save even more lives. To that end, BioFarma Indonesia is developing a new oral rotavirus vaccine, RV3-BB, in partnership with Murdoch Children Research Institute (MCRI) of Australia. Currently undergoing Phase 3 clinical trials in Indonesia, this candidate could be a promising addition to current rotavirus vaccine options. PATH is supporting BioFarma in optimizing the RV3 manufacturing process to improve vaccine yields and supply.
Dr. Vicka Oktaria shared a summary of her team’s research to estimate RV3-BB’s potential for lifesaving impact in Indonesia.*
What is RV3-BB?
Based on a naturally occurring rotavirus strain found in healthy newborn babies, the RV3-BB vaccine is administered orally at birth, providing an opportunity for early protection. (Current rotavirus vaccine schedules recommend the first dose between 6-8 weeks of age.) In Indonesia, a clinical trial found that three doses of RV3-BB vaccine protected 94% of babies from severe rotavirus disease through 12 months of age and 75% through 18 months.
How will RV3-BB impact Indonesian children?
To estimate the direct and indirect benefits of RV3-BB, we began by developing a computational model of pediatric rotavirus transmission. Our model incorporated population dynamics (birth, death, and aging); the number of people living in each household; how people interact with others within and between households; disease status (susceptible, infected, immune); and development of disease (see Picture 1). The model also included rotavirus immunity – the protection against rotavirus infection obtained by transfer of maternal antibodies at birth, natural infection, and/or vaccination.
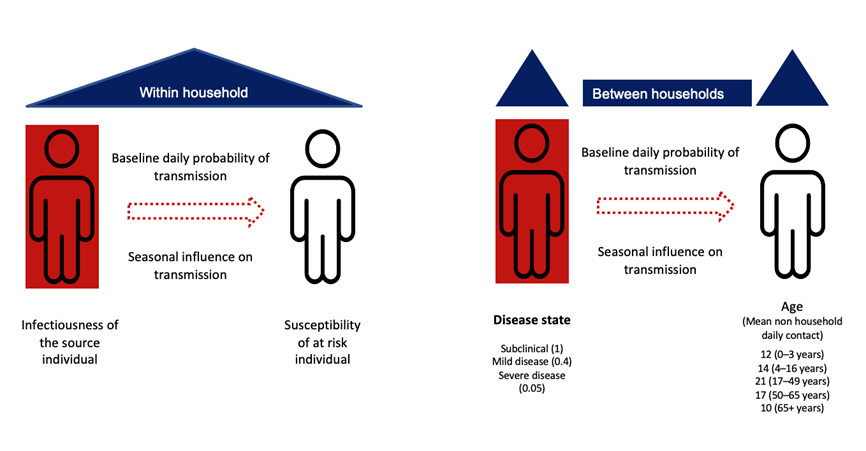
What will happen when RV3-BB is introduced?
Using this agent-based model, we estimated that if 85% of Indonesia’s birth cohort received RV3-BB, we would see a 95% reduction in the incidence of severe rotavirus diarrhea, compared with unvaccinated children. Even if only half of the eligible infant population received RV3-BB, the reduction of infection and disease in children under 12 months of age would be 84%. This impact was seen irrespective if the vaccine was administered with the first dose at birth or at ~8 weeks of age. Adults also indirectly benefited from children receiving RV3-BB and were more than 75% less likely to develop rotavirus diarrhea than in the scenario where there was no childhood vaccination.
Our model results suggest that introduction of the oral neonatal rotavirus vaccine (RV3-BB) into the National Immunisation Program in Indonesia has the potential to make a huge impact on diarrheal disease and hospitalisation due to rotavirus in young children.
* The scientists conducting this research are Dr Vicka Oktaria MPH PhD FRSPH, Center for Child Health-Pediatric Research Office/ Department of Biostatistics, Epidemiology, and Population Health, Universitas Gadjah Mada (UGM), Indonesia; Prof Nic Geard, Computing and Information Systems, the University of Melbourne, Australia; and Prof Julie Bines, MBBS FRACP MD AGAF, Enteric diseases group, Murdoch Children’s Research Institute, Australia
Learn more about rotavirus, the leading cause of severe and deadly diarrhea.


