
Addressing the hidden burden of gut dysfunction

An amino acid–based oral rehydration solution is among the potential solutions to heal gut damage. Photo: PATH/Amy Gottlieb.
Adapted from an article that originally appeared on PATH.org.
The global diarrheal disease burden comes with consequences, and one of the most sinister is environmental enteric dysfunction (EED). Though our knowledge is increasing of its lifelong harm to the most vulnerable children around the world, many aspects of EED remain shrouded in mystery. That’s why I’m thrilled PLOS Neglected Tropical Diseases has launched a new section specifically focused on enteric infections and enteric dysfunction. In the new section’s inaugural editorial, Dr. Judd Walson and I review the current state of the field, and highlight the important knowledge gaps that are imperative for moving forward. Here, I’ll provide a general overview and share two of our promising projects targeting EED at PATH.
Background on Environmental Enteric Dysfunction
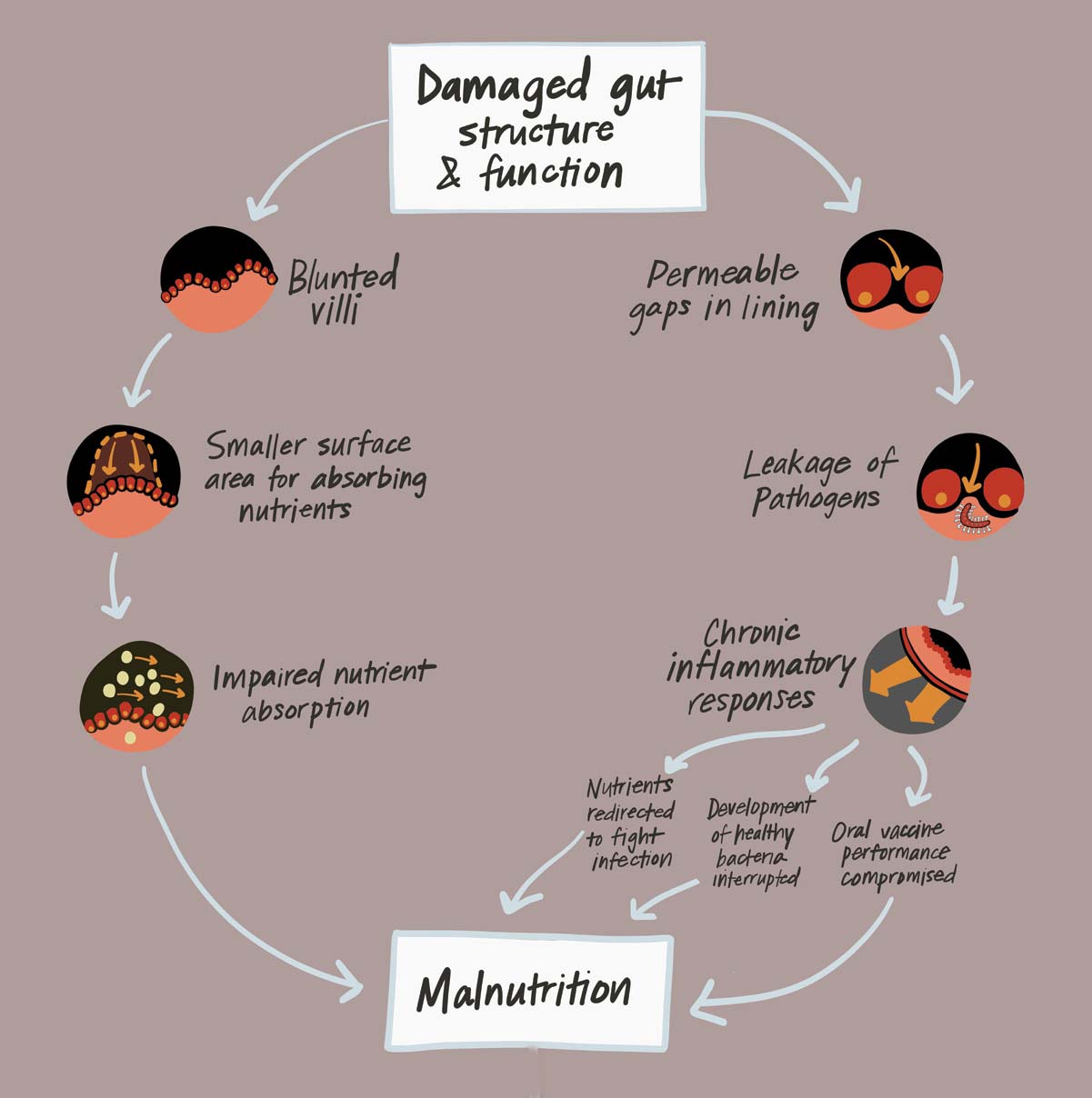
EED is the manifestation of structural gut damage and impaired intestinal function that may result from the combination of malnutrition and persistent gut infections (e.g., viruses and bacteria that cause diarrhea) in early childhood.
EED is virtually ubiquitous in impoverished communities, especially in areas with limited access to clean water, sanitation, and hygiene, where children are constantly exposed to an array of pathogens lurking in the environment. Repeated and chronic gut infections can provoke an inflammatory response in the intestine that results in a cascade of harmful effects.
Nutrients that would typically fuel growth, cognitive development, and a strong immune system can be diverted toward fighting off infection and tissue repair. Chronic gut inflammation appears to cause structural changes that impede the intestine’s ability to absorb nutrients from food. It also interrupts the development of healthy commensal bacteria and compromises the performance of oral vaccines. Combined, these changes increase children’s vulnerability to further infections and risk for growth faltering (i.e., stunting).
Over a lifetime, stunting is linked to diminishing childrens’ ability to reach their full potential. Linked with impaired cognitive development, poor school performance, and ultimately reduced future earnings, stunting and the complex underlying etiology helps to keep entire communities entrenched in a cycle of poverty and poor health.
Unfortunately, the cycle of stunting and poor health can persist for generations. Young girls who experience growth stunting are more likely to suffer from malnutrition as adults. When undernourished women become pregnant, they are at greater risk for premature birth and low birth weight, increasing the likelihood of stunting in their own infants.
PATH is advancing these goals by bringing together cross-sector partners to foster research, and projects aimed at enabling development of therapeutics for minimizing, preventing, or improving intestinal injury in young children.
Multi-micronutrient and EED Assessment Tool (MEEDAT)
To detect the presence of EED in individuals, we need good biomarkers, which (put simply) serve as an indicator of the presence or severity of a disease state (e.g., body temperature for fever, systolic blood pressure for risk of heart disease or stroke). However, the current tools to quantify biomarkers of gut function and micronutrient status are expensive, time-consuming, and labor-intensive. To meet the need for an effective, affordable research tool that enables the measurement of multiple EED biomarkers and micronutrients from children in low-resource settings, PATH has co-developed MEEDAT. MEEDAT is a multiplex assay for quantifying blood-based biomarkers of EED, growth hormone resistance, systemic inflammation, and micronutrient status in children participating in clinical research studies.
Amino acid-based oral rehydration solution as a potential EED intervention
We believe the main goal of an effective EED therapeutic should be to lessen or improve intestinal injury related to chronic and/or recurrent enteric illnesses. Enterade® is an amino acid–based oral rehydration solution (AA-ORS) currently sold in the U.S. as a medical food to manage gut damage and dysfunction due to radiation and chemotherapy. AA-ORS consists of oral rehydration salts and a blend of amino acids that together appear to restore gut function, improve nutrient and electrolyte absorption, and improve barrier integrity. Pre-clinical models demonstrated that an AA-ORS similar to Enterade® increased electrolyte absorption, decreased gut permeability, increased weight gain, and improved survival in irradiated mice with damaged gut. AA-ORS may benefit young children with EED, as this product reduces inflammation and promotes healing of damaged intestinal epithelium. Therefore PATH is evaluating AA-ORS in a pilot study in western Kenya.
The study will help us answer questions about the safety, feasibility, and caregiver acceptability of AA-ORS as a treatment for children aged 12-24 months who are at risk for EED. It will also preliminarily test the impact of the product on biomarkers of gut dysfunction, metabolism, gut inflammation, systemic inflammation, and micronutrient status.
Read the recent EED editorial published in PLOS Neglected Tropical Diseases, and follow the journal for the latest research. If you’re keen to participate in setting the EED research agenda, join the EED working group within the multisectoral Diarrhea Innovations Group. And as always, join the DefeatDD conversation on Facebook, Twitter, Instagram, and LinkedIn.


